- Record: found
- Abstract: found
- Article: found
Musashi1 regulates breast tumor cell proliferation and is a prognostic indicator of poor survival
Read this article at
Abstract
Background
Musashi1 (Msi1) is a conserved RNA-binding protein that regulates the Notch and Wnt pathways, and serves as a stem cell marker in the breast and other tissues. It is unknown how Msi1 relates to other breast cancer markers, whether it denotes tumor initiating cells (TICs), and how it affects gene expression and tumor cell survival in breast cancer cells.
Results
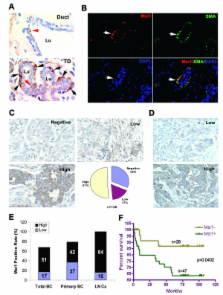
Msi1 expression was analyzed in 20 breast cancer cell lines and in 140 primary breast tumors by western blotting and immunohistochemistry, respectively. Lentivirus RNA interference was used to reduce Msi1 expression in breast cancer cell lines MCF-7 and T47D grown as spheroid cultures and to assess stem cell gene expression and the growth of these cell lines as xenografts. In normal human breast tissue, Msi1 was expressed in 10.6% of myoepithelum and 1.2% of ductal epithelium in the terminal ductal lobular unit (TDLU), whereas, less than 0.05% of ductal epithelium and myoepithelium in large ducts outside the TDLU expressed Msi1. Msi1 was expressed in 55% of the breast cancer cell lines and correlated with ErbB2 expression in 50% of the cell lines. Msi1 was expressed in 68% of primary tumors and in 100% of lymph node metastases, and correlated with 5 year survival. Msi1 was enriched in CD133 + MCF-7 and T47D cells and in spheroid cultures of these cells, and Msi1 'knockdown' (KD) with a lentivirus-expressed shRNA decreased the number and size of spheroid colonies. Msi1 KD reduced Notch1, c-Myc, ErbB2 and pERK1/2 expression, and increased p21 CIP1 expression, which is consistent with known Msi1 target mRNAs. Msi1 KD also reduced the expression of the somatic and embryonic stem cell markers, CD133, Bmi1, Sox2, Nanog and Oct4. Xenografts of MCF-7 and T47D Msi1 KD cells resulted in a marked reduction of tumor growth, reduced Msi1 and Notch1 expression and increased p21 CIP1 expression.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation.
- Record: found
- Abstract: found
- Article: not found