- Record: found
- Abstract: found
- Article: found
Small Molecule Fluorescent Ligands for the Atypical Chemokine Receptor 3 (ACKR3)

Read this article at
Abstract

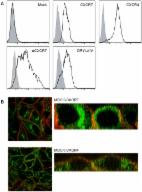
The atypical chemokine receptor 3 (ACKR3) is a receptor that induces cancer progression and metastasis in multiple cell types. Therefore, new chemical tools are required to study the role of ACKR3 in cancer and other diseases. In this study, fluorescent probes, based on a series of small molecule ACKR3 agonists, were synthesized. Three fluorescent probes, which showed specific binding to ACKR3 through a luminescence-based NanoBRET binding assay (p K d ranging from 6.8 to 7.8) are disclosed. Due to their high affinity at the ACKR3, we have shown their application in both competition binding experiments and confocal microscopy studies showing the cellular distribution of this receptor.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
FRED and HYBRID docking performance on standardized datasets.
- Record: found
- Abstract: found
- Article: not found