- Record: found
- Abstract: found
- Article: found
Transforming Growth Factor-β Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts
Read this article at
Abstract
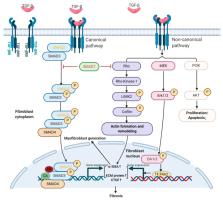
Transforming growth factor-β (TGF-β) signaling is essential in embryo development and maintaining normal homeostasis. Extensive evidence shows that TGF-β activation acts on several cell types, including epithelial cells, fibroblasts, and immune cells, to form a pro-fibrotic environment, ultimately leading to fibrotic diseases. TGF-β is stored in the matrix in a latent form; once activated, it promotes a fibroblast to myofibroblast transition and regulates extracellular matrix (ECM) formation and remodeling in fibrosis. TGF-β signaling can also promote cancer progression through its effects on the tumor microenvironment. In cancer, TGF-β contributes to the generation of cancer-associated fibroblasts (CAFs) that have different molecular and cellular properties from activated or fibrotic fibroblasts. CAFs promote tumor progression and chronic tumor fibrosis via TGF-β signaling. Fibrosis and CAF-mediated cancer progression share several common traits and are closely related. In this review, we consider how TGF-β promotes fibrosis and CAF-mediated cancer progression. We also discuss recent evidence suggesting TGF-β inhibition as a defense against fibrotic disorders or CAF-mediated cancer progression to highlight the potential implications of TGF-β-targeted therapies for fibrosis and cancer.
Related collections
Most cited references167
- Record: found
- Abstract: found
- Article: not found
The Emerging Hallmarks of Cancer Metabolism.

- Record: found
- Abstract: found
- Article: found
A framework for advancing our understanding of cancer-associated fibroblasts
- Record: found
- Abstract: found
- Article: not found