- Record: found
- Abstract: found
- Article: found
Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer

Read this article at
Abstract
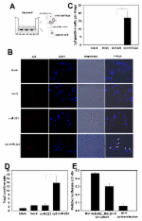
Cancer-associated fibroblasts (CAFs) are the major components of the tumor microenvironment. They may drive tumor progression, although the mechanisms involved are still poorly understood. Exosomes have emerged as important mediators of intercellular communication in cancer. They mediate horizontal transfer of microRNAs (miRs), mRNAs and proteins, thus affecting breast cancer progression. Differential expression profile analysis identified three miRs (miRs -21, -378e, and -143) increased in exosomes from CAFs as compared from normal fibroblasts. Immunofluorescence indicated that exosomes may be transferred from CAFs to breast cancer cells, releasing their cargo miRs. Breast cancer cells (BT549, MDA-MB-231, and T47D lines) exposed to CAF exosomes or transfected with those miRs exhibited a significant increased capacity to form mammospheres, increased stem cell and epithelial-mesenchymal transition (EMT) markers, and anchorage-independent cell growth. These effects were reverted by transfection with anti-miRs. Similarly to CAF exosomes, normal fibroblast exosomes transfected with miRs -21, -378e, and -143 promoted the stemness and EMT phenotype of breast cancer cells. Thus, we provided evidence for the first time of the role of CAF exosomes and their miRs in the induction of the stemness and EMT phenotype in different breast cancer cell lines. Indeed, CAFs strongly promote the development of an aggressive breast cancer cell phenotype.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity.
- Record: found
- Abstract: found
- Article: found
Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells
- Record: found
- Abstract: found
- Article: not found