- Record: found
- Abstract: found
- Article: found
NAD + homeostasis in human health and disease
Read this article at
Abstract
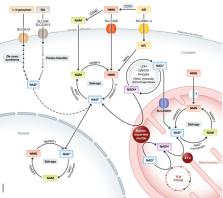
Depletion of nicotinamide adenine dinucleotide (NAD +), a central redox cofactor and the substrate of key metabolic enzymes, is the causative factor of a number of inherited and acquired diseases in humans. Primary deficiencies of NAD + homeostasis are the result of impaired biosynthesis, while secondary deficiencies can arise due to other factors affecting NAD + homeostasis, such as increased NAD + consumption or dietary deficiency of its vitamin B3 precursors. NAD + depletion can manifest in a wide variety of pathological phenotypes, ranging from rare inherited defects, characterized by congenital malformations, retinal degeneration, and/or encephalopathy, to more common multifactorial, often age‐related, diseases. Here, we discuss NAD + biochemistry and metabolism and provide an overview of the etiology and pathological consequences of alterations of the NAD + metabolism in humans. Finally, we discuss the state of the art of the potential therapeutic implications of NAD + repletion for boosting health as well as treating rare and common diseases, and the possibilities to achieve this by means of the different NAD +‐enhancing agents.
Abstract
Related collections
Most cited references146
- Record: found
- Abstract: found
- Article: not found
Poly(ADP-ribose): novel functions for an old molecule.
- Record: found
- Abstract: found
- Article: not found
NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus.
- Record: found
- Abstract: found
- Article: not found
