- Record: found
- Abstract: found
- Article: not found
Melanopsin-Driven Light Adaptation in Mouse Vision
Read this article at
Summary
Background
In bright light, mammals use a distinct photopigment (melanopsin) to measure irradiance for centrally mediated responses such as circadian entrainment. We aimed to determine whether the information generated by melanopsin is also used by the visual system as a signal for light adaptation. To this end, we compared retinal and thalamic responses to a range of artificial and natural visual stimuli presented using spectral compositions that either approximate the mouse’s experience of natural daylight (“daylight”) or are selectively depleted of wavelengths to which melanopsin is most sensitive (“mel-low”).
Results
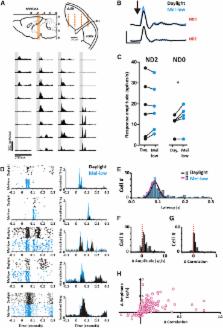
We found reproducible and reversible changes in the flash electroretinogram between daylight and mel-low. Simultaneous recording in the dorsal lateral geniculate nucleus (dLGN) revealed that these reflect changes in feature selectivity of visual circuits in both temporal and spatial dimensions. A substantial fraction of units preferred finer spatial patterns in the daylight condition, while the population of direction-sensitive units became tuned to faster motion. The dLGN contained a richer, more reliable encoding of natural scenes in the daylight condition. These effects were absent in mice lacking melanopsin.
Conclusions
The feature selectivity of many neurons in the mouse dLGN is adjusted according to a melanopsin-dependent measure of environmental brightness. These changes originate, at least in part, within the retina. Melanopsin performs a role analogous to a photographer’s light meter, providing an independent measure of irradiance that determines optimal setting for visual circuits.
Highlights
Abstract
Allen et al. show that aspects of light adaptation rely on a measure of brightness provided by melanopsin, the retina’s most recently discovered photoreceptor. Recordings from the mouse thalamus show that melanopsin controls the types of visual features that excite single neurons, allowing a richer, more reliable representation of natural scenes.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN.
- Record: found
- Abstract: found
- Article: not found
Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice.
- Record: found
- Abstract: found
- Article: not found
