- Record: found
- Abstract: found
- Article: found
Atlantic salmon skin barrier functions gradually enhance after seawater transfer
Read this article at
Abstract
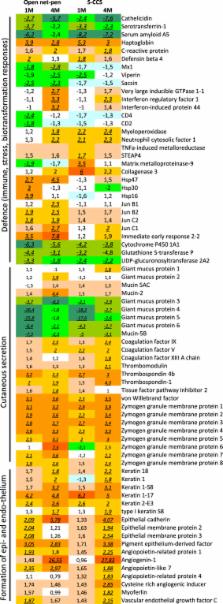
Atlantic salmon farming operates with high production intensities where skin integrity is recognized as a central factor and indicator for animal health and welfare. In the described trial, the skin development and its immune status in healthy Atlantic salmon reared in two different systems, a traditional open net-pen system and a semi-closed containment system, were investigated. Freshwater smolts were compared to post-smolts after 1 and 4 months in seawater. Growth performance, when adjusted for temperature, was equal between the systems. Skin analyses, including epidermis and dermis, showed that thickness and mucus cell numbers increased in pace with the growth and time post seawater transfer (PST). Gene expression changes suggested similar processes with development of connective tissue, formation of extracellular matrix and augmented cutaneous secretion, changes in mucus protein composition and overall increased immune activity related to gradually enforced protection against pathogens. Results suggest a gradual morphological development in skin with a delayed recovery of immune functions PST. It is possible that Atlantic salmon could experience increased susceptibility to infectious agents and risk of diseases during the first post-smolt period.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut.

- Record: found
- Abstract: found
- Article: found
Local and systemic gene expression responses of Atlantic salmon (Salmo salar L.) to infection with the salmon louse (Lepeophtheirus salmonis)
- Record: found
- Abstract: found
- Article: not found