- Record: found
- Abstract: found
- Article: found
Early Changes Over Time in the Radiographic Assessment of Lung Edema Score Are Associated With Survival in ARDS
Read this article at
Abstract
Background
The Radiographic Assessment of Lung Edema (RALE) score is associated with the severity of ARDS, and treatments targeted at reducing pulmonary edema such as conservative fluid management cause a reduction in RALE score over time.
Research Question
Are early changes in RALE score over time associated with survival in patients with ARDS?
Study Design and Methods
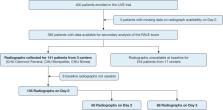
Data from patients enrolled in three centers in the Lung Imaging for Ventilation sEtting in ARDS (LIVE) trial with available chest radiographs at baseline (day 0) and days 2 or 3 were used. The RALE was scored by two independent reviewers. The primary end point was death by day 90, considering RALE score both at baseline and as a time-varying covariate in a marginal Cox survival model.
Results
RALE was scored from 135, 64, and 88 radiographs on days 0, 2, and 3, respectively. Both baseline RALE (hazard ratio [HR] for each one-point increment, 1.04; 95% CI, 1.01-1.08; P = .006) and the change in RALE over time (HR for each one-point decrease per unit of time, 0.99; 95% CI, 0.99-0.99; P = .03) were associated with death by day 90, even after adjustment for age, sex, BMI, Simplified Acute Physiology Score II, vasopressor use, and total volume of fluids received since study entry.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Acute respiratory distress syndrome: the Berlin Definition.
- Record: found
- Abstract: found
- Article: not found
A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study.
- Record: found
- Abstract: found
- Article: not found
Comparison of two fluid-management strategies in acute lung injury.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.