- Record: found
- Abstract: found
- Article: found
New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis
Read this article at
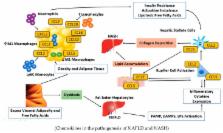
Abstract
NASH is becoming increasingly common worldwide because of the growing global prevalence of obesity and consequently NAFLD. Unfortunately, the mechanism of progression of NAFLD to NASH and then cirrhosis is not completely understood. Several factors, including insulin resistance, inflammation, oxidative stress, lipotoxicity, and bile acid (BA) toxicity, have been reported to be associated with NASH progression. The release of fatty acids from dysfunctional and insulin-resistant adipocytes results in lipotoxicity, which is caused by the ectopic accumulation of triglyceride-derived toxic metabolites and the subsequent activation of inflammatory pathways, cellular dysfunction, and lipoapoptosis. Adipose tissue (AT), especially visceral AT, comprises multiple cell populations that produce adipokines and insulin-like growth factor, plus macrophages and other immune cells that stimulate the development of lipotoxic liver disease. These biomolecules have been recently linked with many digestive diseases and gastrointestinal malignancies such as hepatocellular carcinoma. This made us question what role lipotoxicity has in the natural history of liver fibrosis. Therefore, this review focuses on the close relationship between AT and NASH. A good comprehension of the pathways that are related to dysregulated AT, metabolic dysfunction, and hepatic lipotoxicity will result in the development of prevention strategies and promising therapeutics for patients with NASH.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study.
- Record: found
- Abstract: found
- Article: not found
Regulation of lipolysis in adipocytes.
- Record: found
- Abstract: found
- Article: not found