- Record: found
- Abstract: found
- Article: found
Cytoskeletal organization of axons in vertebrates and invertebrates
Read this article at
Abstract
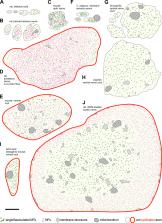
Five decades of ultrastructural studies of axons are reviewed and reinterpreted on the basis of current mechanistic knowledge, revealing microtubule bundles as the essential common architectural element of vertebrate and invertebrate axons.
Abstract
The maintenance of axons for the lifetime of an organism requires an axonal cytoskeleton that is robust but also flexible to adapt to mechanical challenges and to support plastic changes of axon morphology. Furthermore, cytoskeletal organization has to adapt to axons of dramatically different dimensions, and to their compartment-specific requirements in the axon initial segment, in the axon shaft, at synapses or in growth cones. To understand how the cytoskeleton caters to these different demands, this review summarizes five decades of electron microscopic studies. It focuses on the organization of microtubules and neurofilaments in axon shafts in both vertebrate and invertebrate neurons, as well as the axon initial segments of vertebrate motor- and interneurons. Findings from these ultrastructural studies are being interpreted here on the basis of our contemporary molecular understanding. They strongly suggest that axon architecture in animals as diverse as arthropods and vertebrates is dependent on loosely cross-linked bundles of microtubules running all along axons, with only minor roles played by neurofilaments.
Related collections
Most cited references300
- Record: found
- Abstract: found
- Article: not found
Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.
- Record: found
- Abstract: found
- Article: not found
Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation.
- Record: found
- Abstract: found
- Article: not found