- Record: found
- Abstract: found
- Article: not found
Aqueous cannabidiol β-cyclodextrin complexed polymeric micelle nasal spray to attenuate in vitro and ex-vivo SARS-CoV-2-induced cytokine storms
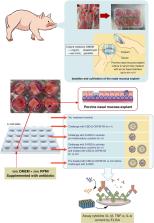
Graphical abstract
Abstract
Cannabidiol (CBD) has a number of biological effects by acting on the cannabinoid receptors CB 1 and CB 2. CBD may be involved in anti-inflammatory processes via CB 1 and CB 2 receptors, resulting in a decrease of pro-inflammatory cytokines. However, CBD's poor aqueous solubility is a major issue in pharmaceutical applications. The aim of the present study was to develop and evaluate a CBD nasal spray solution. A water-soluble CBD was prepared by complexation with β-cyclodextrin (β-CD) at a stoichiometric ratio of 1:1 and forming polymeric micelles using poloxamer 407. The mixture was then lyophilized and characterized using FT-IR, DSC, and TGA. CBD-β-CD complex-polymeric micelles were formulated for nasal spray drug delivery. The physicochemical properties of the CBD-β-CD complex-polymeric micelle nasal spray solution (CBD-β-CDPM-NS) were assessed. The results showed that the CBD content in the CBD-β-CD complex polymeric micelle powder was 102.1 ± 0.5%. The CBD-β-CDPM-NS was a clear colorless isotonic solution. The particle size, zeta potential, pH value, and viscosity were 111.9 ± 0.7 nm, 0.8 ± 0.1 mV, 6.02 ± 0.02, and 12.04 ± 2.64 cP, respectively. This formulation was stable over six months at ambient temperature. The CBD from CBD-β-CDPM-NS rapidly released to 100% within 1 min. Ex-vivo permeation studies of CBD-β-CDPM-NS through porcine nasal mucosa revealed a permeation rate of 4.8 μg/cm 2/min, which indicated that CBD was effective in penetrating nasal epithelial cells. CBD-β-CDPM-NS was tested for its efficacy and safety in terms of cytokine production from nasal immune cells and toxicity to nasal epithelial cells. The CBD-β-CDPM-NS was not toxic to nasal epithelial at the concentration of CBD equivalent to 3.125-50 μg/mL. When the formulation was subjected to bioactivity testing against monocyte-like macrophage cells, it proved that the CBD-β-CDPM-NS has the potential to inhibit inflammatory cytokines. CBD-β-CDPM-NS demonstrated the formulation's ability to reduce the cytokine produced by S-RBD stimulation in ex vivo porcine nasal mucosa in both preventative and therapeutic modes.
Related collections
Most cited references58
- Record: found
- Abstract: not found
- Article: not found
A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding
- Record: found
- Abstract: found
- Article: not found
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.
- Record: found
- Abstract: found
- Article: not found
COVID-19 cytokine storm: The anger of inflammation
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
