- Record: found
- Abstract: found
- Article: found
Requirements for the differentiation of innate T-bet high memory-phenotype CD4 + T lymphocytes under steady state
Read this article at
Abstract
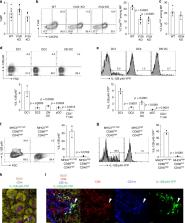
CD4 + T lymphocytes consist of naïve, antigen-specific memory, and memory-phenotype (MP) cell compartments at homeostasis. We recently showed that MP cells exert innate-like effector function during host defense, but whether MP CD4 + T cells are functionally heterogeneous and, if so, what signals specify the differentiation of MP cell subpopulations under homeostatic conditions is still unclear. Here we characterize MP lymphocytes as consisting of T-bet high, T-bet low, and T-bet − subsets, with innate, Th1-like effector activity exclusively associated with T-bet high cells. We further show that the latter population depends on IL-12 produced by CD8α + type 1 dendritic cells (DC1) for its differentiation. Finally, our data demonstrate that this tonic IL-12 production requires TLR-MyD88 signaling independent of foreign agonists, and is further enhanced by CD40-CD40L interactions between DC1 and CD4 + T lymphocytes. We propose that optimal differentiation of T-bet high MP lymphocytes at homeostasis is driven by self-recognition signals at both the DC and Tcell levels.
Abstract
CD4 + T cells contain a T-bet high memory-phenotype (MP) population with innate-like functions. Here the authors characterize the requirements for their differentiation at homeostasis and identify a function for IL-12 that is tonically produced by type 1 dendritic cells in an MyD88- and CD40-dependent, but foreign PAMP-independent manner.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity.
- Record: found
- Abstract: found
- Article: not found
Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components
- Record: found
- Abstract: found
- Article: not found