- Record: found
- Abstract: found
- Article: found
Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst
Read this article at
Abstract
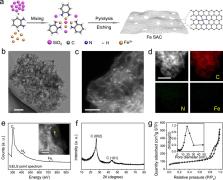
Electrochemically converting nitrate, a widespread water pollutant, back to valuable ammonia is a green and delocalized route for ammonia synthesis, and can be an appealing and supplementary alternative to the Haber-Bosch process. However, as there are other nitrate reduction pathways present, selectively guiding the reaction pathway towards ammonia is currently challenged by the lack of efficient catalysts. Here we report a selective and active nitrate reduction to ammonia on Fe single atom catalyst, with a maximal ammonia Faradaic efficiency of ~ 75% and a yield rate of up to ~ 20,000 μg h −1 mg cat. −1 (0.46 mmol h −1 cm −2). Our Fe single atom catalyst can effectively prevent the N-N coupling step required for N 2 due to the lack of neighboring metal sites, promoting ammonia product selectivity. Density functional theory calculations reveal the reaction mechanisms and the potential limiting steps for nitrate reduction on atomically dispersed Fe sites.
Abstract
Developing green and delocalized routes for ammonia synthesis is highly important but still very challenging. Here the authors report an efficient ammonia synthesis process via nitrate reduction to ammonia on Fe single atom catalyst.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: not found
QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials.
- Record: found
- Abstract: not found
- Article: not found
Heterogeneous single-atom catalysis
- Record: found
- Abstract: found
- Article: not found