- Record: found
- Abstract: found
- Article: found
Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study

Read this article at
Abstract
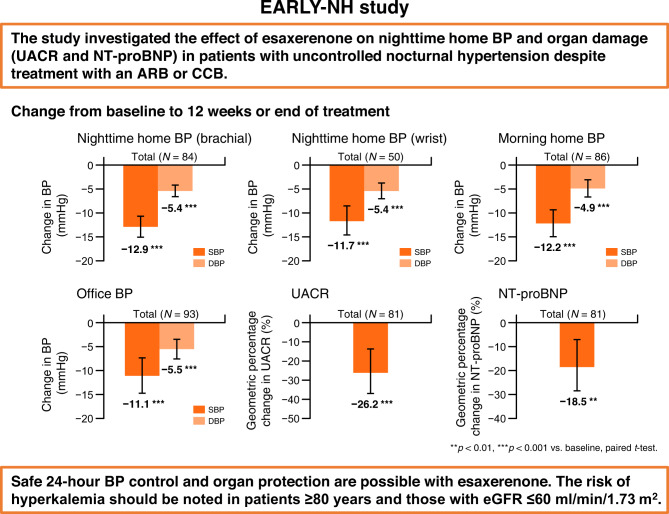
There is limited evidence on the blood pressure (BP)-lowering effect of esaxerenone on home BP, including nighttime BP. Using two newly developed nocturnal home BP monitoring devices (brachial and wrist), this multicenter, open-label, prospective study investigated the nighttime home BP-lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension being treated with an angiotensin receptor blocker (ARB) or calcium-channel blocker (CCB). In total, 101 patients were enrolled. During the 12-week study period, change in nighttime home systolic/diastolic BP from baseline to end of treatment measured by the brachial device was −12.9/−5.4 mmHg in the total population and −16.2/−6.6 and −10.0/−4.4 mmHg in the ARB and CCB subcohorts, respectively (all p < 0.001). For the wrist device, the change was −11.7/−5.4 mmHg in the total population and −14.6/−6.2 and −8.3/−4.5 mmHg in each subcohort, respectively (all p < 0.001). Similar significant reductions were shown for morning and bedtime home BP and office BP. Urinary albumin-to-creatinine ratio, N-terminal pro-brain natriuretic peptide, and cardio-ankle vascular index improved in the total population and each subcohort. Incidences of treatment-emergent adverse events (TEAEs) and drug-related TEAEs were 38.6% and 16.8%, respectively; most were mild or moderate. The most frequent drug-related TEAEs were associated with serum potassium elevation (hyperkalemia, 9.9%; blood potassium increased, 3.0%); however, no new safety concerns were raised. Esaxerenone was effective in lowering nighttime home BP as well as morning and bedtime home BP and office BP, safe, and showed organ-protective effects in patients with uncontrolled nocturnal hypertension. Caution is warranted regarding elevated serum potassium levels.
Abstract
This study investigated the effect of esaxerenone on nighttime home BP and organ damage (UACR and NT-proBNP) in patients with uncontrolled nocturnal hypertension despite treatment with an ARB or CCB. Our results show that safe 24-h BP control and organ protection are possible with esaxerenone.
Related collections
Most cited references45
- Record: found
- Abstract: not found
- Article: not found
The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019)
- Record: found
- Abstract: found
- Article: not found
Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials
- Record: found
- Abstract: found
- Article: not found