- Record: found
- Abstract: found
- Article: found
Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells
Read this article at
Abstract
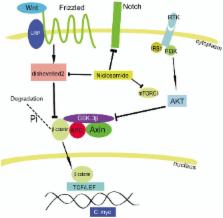
Niclosamide, an oral antihelminthic drug, has been used to treat tapeworm infection for about 50 years. Niclosamide is also used as a molluscicide for water treatment in schistosomiasis control programs. Recently, several groups have independently discovered that niclosamide is also active against cancer cells, but its precise mechanism of antitumor action is not fully understood. Evidence supports that niclosamide targets multiple signaling pathways (NF-κB, Wnt/β-catenin, Notch, ROS, mTORC1, and Stat3), most of which are closely involved with cancer stem cells. The exciting advances in elucidating the antitumor activity and the molecular targets of this drug will be discussed. A method for synthesizing a phosphate pro-drug of niclosamide is provided. Given its potential antitumor activity, clinical trials for niclosamide and its derivatives are warranted for cancer treatment.
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: not found
Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene.
- Record: found
- Abstract: not found
- Article: not found