- Record: found
- Abstract: found
- Article: found
Potassium binders for patients with heart failure? The real enlightenment of the DIAMOND trial
Read this article at
Graphical Abstract
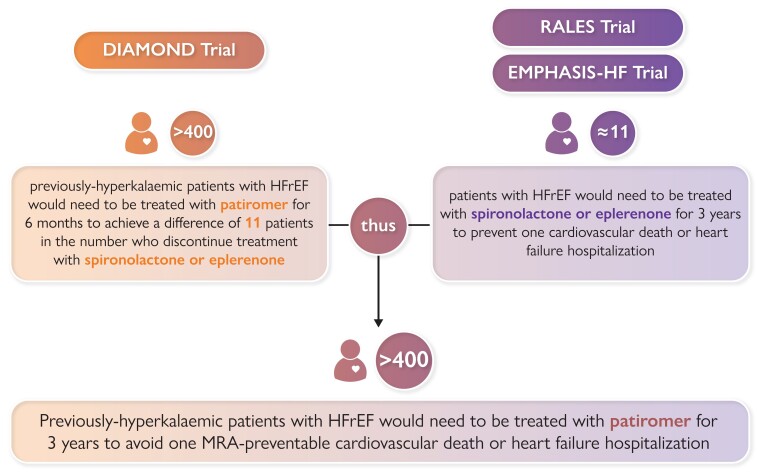
Estimation of Number-Needed-to-Treat with Patiromer for 3 years to Prevent One Cardiovascular Death or Hospitalization for Heart failure in Patients with Heart Failure and a Reduced Ejection Fraction. Estimates of number-needed-to-treat for the RALES and EMPHASIS-HF trials are derived from calculations published by Ferreira et al. 13 Estimates for the DIAMOND trial are derived from results presented by Butler et al. 11 Abbreviations: MRA = mineralocorticoid receptor antagonist. HFrEF = heart failure and a reduced ejection fraction.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators.
- Record: found
- Abstract: found
- Article: not found
Eplerenone in patients with systolic heart failure and mild symptoms.
- Record: found
- Abstract: found
- Article: not found