- Record: found
- Abstract: found
- Article: found
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Read this article at
Abstract
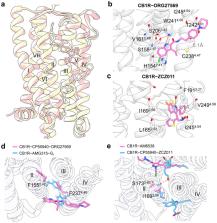
G protein-coupled receptors (GPCRs), the largest family of human membrane proteins and an important class of drug targets, play a role in maintaining numerous physiological processes. Agonist or antagonist, orthosteric effects or allosteric effects, and biased signaling or balanced signaling, characterize the complexity of GPCR dynamic features. In this study, we first review the structural advancements, activation mechanisms, and functional diversity of GPCRs. We then focus on GPCR drug discovery by revealing the detailed drug-target interactions and the underlying mechanisms of orthosteric drugs approved by the US Food and Drug Administration in the past five years. Particularly, an up-to-date analysis is performed on available GPCR structures complexed with synthetic small-molecule allosteric modulators to elucidate key receptor-ligand interactions and allosteric mechanisms. Finally, we highlight how the widespread GPCR-druggable allosteric sites can guide structure- or mechanism-based drug design and propose prospects of designing bitopic ligands for the future therapeutic potential of targeting this receptor family.
Related collections
Most cited references470

- Record: found
- Abstract: found
- Article: found
Highly accurate protein structure prediction with AlphaFold
- Record: found
- Abstract: found
- Article: not found
The Protein Data Bank.
- Record: found
- Abstract: found
- Article: found