- Record: found
- Abstract: found
- Article: found
Radiofrequency ablation (RFA) vs. argon plasma coagulation (APC) for the management of gastric antral vascular ectasia (GAVE) in patients with and without cirrhosis: results from a retrospective analysis of a large cohort of patients treated at a single center
Read this article at
Abstract
Introduction and study aims
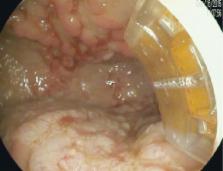
Gastric antral vascular ectasia (GAVE) is a mucosal abnormality associated with multiple conditions, most notably cirrhosis and systemic sclerosis, that causes indolent gastrointestinal bleeding. It is primarily managed with endoscopic therapy. Traditionally, GAVE is endoscopically ablated using argon plasma coagulation (APC) but radiofrequency ablation (RFA) is emerging as an alternative modality. No prior comparison of the 2 modalities has been published.
Patients and methods
After receiving IRB approval, we reviewed our electronic health records to identify all patients who underwent endoscopic evaluation for GAVE between January, 2011 and October, 2016. We compared important variables between APC and RFA, as well as between cirrhosis and non-cirrhosis, using the Chi-square test and the Wilcoxon two-sample test as appropriate.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Diagnosis and management of gastric antral vascular ectasia.
- Record: found
- Abstract: found
- Article: found
Gastric Antral Vascular Ectasia (GAVE): An Update on Clinical Presentation, Pathophysiology and Treatment
- Record: found
- Abstract: found
- Article: not found