- Record: found
- Abstract: not found
- Article: not found
A decade of nutraceutical patents: where are we now in 2018?
November 22 2018
November 29 2018
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
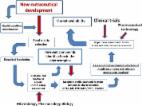
In the last 10 years, nutraceuticals have grown in interest to researchers, industry,
and consumers and are now familiar in the collective imagination as a tool for preventing
the onset of a disease. Often nutraceuticals are confused with biologically active
phytochemicals/botanicals which can have health benefits. This is a misunderstanding
however as the term nutraceutical refers to a product that must have a beneficial
effect on health proven by clinical testing. Areas covered: A search has been performed
on both recent patents and the literature regarding nutraceuticals focusing on the
beneficial and proven health effects on pathological conditions to give an overview
of the state-of-the-art developments in this area. Patents and literature data addressing
specific pathological conditions are discussed. Expert opinion: Nutraceuticals represent
a challenge for the future of drug-based pharmacotherapy, and, at the same time, are
a powerful tool for the prevention of chronic disease. They are not proposed as an
alternative to drugs, but instead, can be helpful to complement a pharmacological
therapy and prevent the onset of chronic diseases in subjects who do not qualify for
conventional pharmacological treatment.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Efficacy of berberine in patients with type 2 diabetes mellitus.
Jun Yin, Huili Xing, Jianping Ye (2008)
- Record: found
- Abstract: found
- Article: not found
Role of nutraceuticals in human health.
Lipi Das, Eshani Bhaumik, Utpal Raychaudhuri … (2012)
- Record: found
- Abstract: found
- Article: found