- Record: found
- Abstract: found
- Article: found
Frontiers in Drug Research and Development for Inflammatory Bowel Disease
Read this article at
Abstract
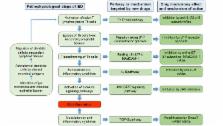
Inflammatory bowel disease (IBD) is idiopathic, lifelong, immune-mediated diseases, for which curative therapies are not yet available. In the last 15 years, the introduction of monoclonal antibodies targeting tumor necrosis factor-α, a cytokine playing a key role in bowel inflammation, has revolutionized treatment paradigms for IBD. Despite their proven long-term efficacy, however, many patients do not respond or progressively lose response to these drugs. Major advances of knowledge in immunology and pathophysiology of intestinal inflammatory processes have made possible the identification of new molecular targets for drugs, thus opening several new potential therapeutic opportunities for IBD. The abnormal response of intestinal immunity to unknown antigens leads to the activation of T helper lymphocytes and triggers the inflammatory cascade. Sphingosine 1-phosphate receptor agonists negatively modulate the egress of lymphocytes, inducted by antigen-presenting cells, from secondary lymphoid tissues to intestinal wall. Leukocyte adhesion inhibitors (both anti-integrin and anti-Mucosal Vascular Addressin Cell Adhesion Molecule 1) interfere with the tissue homing processes. Activated T helper lymphocytes increase the levels of pro-inflammatory cytokines, such as interleukin 12, 23, and 6, offering several potential pharmacological interventions. The Janus kinases, intracellular enzymes mediating the transduction of several cytokine signals, are other explored targets for treating immune-mediated diseases. Finally, the impact of modulating Smad7 pathway, which is responsible for the down-regulation of the immunosuppressive cytokine transforming growth factor-β signaling, is currently under investigation. The purpose of this review is to discuss the most promising molecules in late-stage clinical development, with a special emphasis on pharmacological properties.
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
Ulcerative colitis
- Record: found
- Abstract: found
- Article: not found