- Record: found
- Abstract: found
- Article: found
Targeting Cytokine Signaling and Lymphocyte Traffic via Small Molecules in Inflammatory Bowel Disease: JAK Inhibitors and S1PR Agonists
Read this article at
Abstract
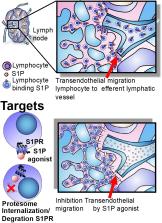
The inflammatory Bowel diseases (IBDs) are a chronic, relapsing inflammatory diseases of the gastrointestinal tract with heterogeneous behavior and prognosis. The introduction of biological therapies including anti-TNF, anti-IL-12/23, and anti-integrins, has revolutionized the treatment of IBD, but these drugs are not universally effective. Due to the complex molecular structures of biologics, they are uniformly immunogenic. New discoveries concerning the underlying mechanisms involved in the pathogenesis of IBD have allowed for progress in the development of new treatment options. The advantage of small molecules (SMs) over biological therapies includes their lack of immunogenicity, short half-life, oral administration, and low manufacturing cost. Among these, the Janus Kinases (JAKs) inhibition has emerged as a novel strategy to modulate downstream cytokine signaling during immune-mediated diseases. These drugs target various cytokine signaling pathways that participate in the pathogenesis of IBD. Tofacitinib, a JAK inhibitor targeting predominantly JAK1 and JAK3, has been approved for the treatment of ulcerative colitis (UC), and there are other specific JAK inhibitors under development that may be effective in Crohn’s. Similarly, the traffic of lymphocytes can now be targeted by another SM. Sphingosine-1-phosphate receptor (S1PR) agonism is a novel strategy that acts, in part, by interfering with lymphocyte recirculation, through blockade of lymphocyte egress from lymph nodes. S1PR agonists are being studied in IBD and other immune-mediated disorders. This review will focus on SM drugs approved and under development, including JAK inhibitors (tofacitinib, filgotinib, upadacitinib, peficitinib) and S1PR agonists (KRP-203, fingolimod, ozanimod, etrasimod, amiselimod), and their mechanism of action.
Related collections
Most cited references70
- Record: found
- Abstract: not found
- Article: not found
Inflammatory bowel disease.
- Record: found
- Abstract: found
- Article: not found
Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis
- Record: found
- Abstract: found
- Article: not found