- Record: found
- Abstract: found
- Article: found
Real-world experience of arbidol for Omicron variant of SARS-CoV-2
Read this article at
Abstract
Background
At a crucial time with the rapid spread of Omicron severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus variant globally, we conducted a study to evaluate the efficacy and safety of arbidol tablets in the treatment of this variant.
Methods
From Mar 26 to April 26, 2022, we conducted a prospective, open-labeled, controlled, and investigator-initiated trial involving adult patients with confirmed Omicron variant infection. Patients with asymptomatic or mild clinical status were stratified 1:2 to receive either standard-of-care (SOC) or SOC plus arbidol tablets (oral administration of 200 mg per time, three times a day for 5 days). The primary endpoint was the negative conversion rate within the first week.
Results
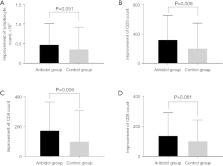
A total of 367 patients were enrolled in the study; 246 received arbidol tablet treatment, and 121 were in the control group. The negative conversion rate of SARS-CoV-2 within the first week in patients receiving arbidol tablets was significantly higher than that of the SOC group [47.2% (116/246) vs. 35.5% (43/121); odds ratio (OR), 1.619; 95% confidence interval (CI): 1.034–2.535; P=0.035]. Compared to those in the SOC group, patients receiving arbidol tablets had a shorter negative conversion time [median 8.3 vs. 10.0 days; hazard ratio (HR), 0.645; 95% CI: 0.516–0.808; P<0.001], and a shorter duration of hospitalization (median 11.4 vs. 13.7 days; HR, 1.214; 95% CI: 0.966–1.526; P<0.001). Moreover, the addition of arbidol tablets led to better recovery of declined blood lymphocytes, CD3 +, CD4 +, and CD8 + cell counts. The most common adverse event (AE) was transaminase elevation in patients treated with arbidol tablets (3/246, 1.2%). No one withdrew from the study due to AEs or disease progression.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections
- Record: found
- Abstract: found
- Article: found