- Record: found
- Abstract: found
- Article: found
Cdc42 regulates junctional actin but not cell polarization in the Caenorhabditis elegans epidermis
Read this article at
Abstract
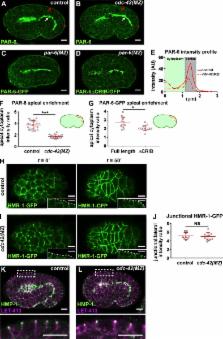
Adherens junction remodeling allows changes in cell shape and position. Zilberman et al. show through live imaging that CDC-42 is dispensable for epithelial cell polarization, but its RhoGAP-regulated activity is needed to control junctional actin organization during embryo elongation.
Abstract
During morphogenesis, adherens junctions (AJs) remodel to allow changes in cell shape and position while preserving adhesion. Here, we examine the function of Rho guanosine triphosphatase CDC-42 in AJ formation and regulation during Caenorhabditis elegans embryo elongation, a process driven by asymmetric epidermal cell shape changes. cdc-42 mutant embryos arrest during elongation with epidermal ruptures. Unexpectedly, we find using time-lapse fluorescence imaging that cdc-42 is not required for epidermal cell polarization or junction assembly, but rather is needed for proper junctional actin regulation during elongation. We show that the RhoGAP PAC-1/ARHGAP21 inhibits CDC-42 activity at AJs, and loss of PAC-1 or the interacting linker protein PICC-1/CCDC85A-C blocks elongation in embryos with compromised AJ function. pac-1 embryos exhibit dynamic accumulations of junctional F-actin and an increase in AJ protein levels. Our findings identify a previously unrecognized molecular mechanism for inhibiting junctional CDC-42 to control actin organization and AJ protein levels during epithelial morphogenesis.
Related collections
Most cited references85
- Record: found
- Abstract: found
- Article: not found
Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences.
- Record: found
- Abstract: found
- Article: not found