- Record: found
- Abstract: found
- Article: found
ZIKV infection induces robust Th1-like Tfh cell and long-term protective antibody responses in immunocompetent mice
Read this article at
Abstract
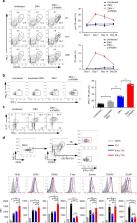
Induction of long-lived antibody responses during infection or vaccination is often essential for subsequent protection, but the relative contributions of T follicular helper (Tfh) cells and T helper 1 (Th1) cells for induction of antigen specific antibody responses to viruses are unclear. Here, we establish an acute Zika virus (ZIKV) infection model in immunocompetent mice, and show that ZIKV infection elicits robust Th1-like Tfh cell and protective antibody responses. While these Th1-like Tfh cells share phenotypic and transcriptomic profiles with both Tfh and Th1 cells, they also have unique surface markers and gene expression characteristics, and are dependent on T-bet for their development. Th1-like Tfh cells, but not Th1 cells, are essential for class switching of ZIKV-specific IgG2c antibodies and maintenance of long-term neutralizing antibody responses. Our study suggests that specific modulation of the Th1-like Tfh cell response during infection or vaccination may augment the induction of antiviral antibody response to ZIKV and other viruses.
Abstract
Here, the authors show that Zika virus (ZIKV) infection induces Th1-like Tfh cells that depend on T-bet for their development and are essential for class switching of ZIKV-specific IgG2c antibodies and maintenance of long-term neutralizing antibody responses.
Related collections
Most cited references19

- Record: found
- Abstract: found
- Article: found
Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses
- Record: found
- Abstract: found
- Article: not found
A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models
- Record: found
- Abstract: found
- Article: not found