- Record: found
- Abstract: found
- Article: found
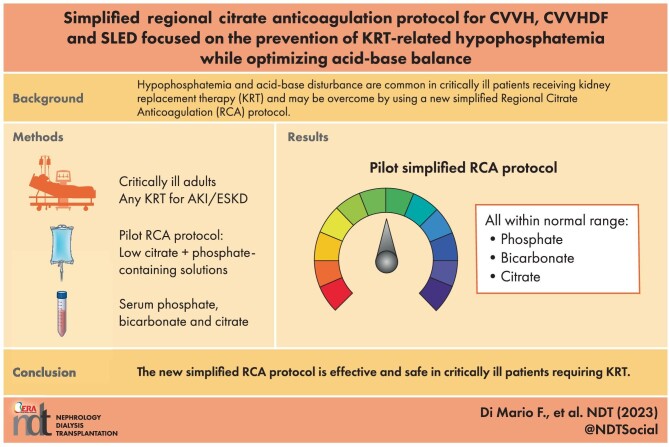
Simplified regional citrate anticoagulation protocol for CVVH, CVVHDF and SLED focused on the prevention of KRT-related hypophosphatemia while optimizing acid-base balance
Read this article at
ABSTRACT
Background
Hypophosphatemia is a common electrolyte disorder in critically ill patients undergoing prolonged kidney replacement therapy (KRT). We evaluated the efficacy and safety of a simplified regional citrate anticoagulation (RCA) protocol for continuous venovenous hemofiltration (CVVH), continuous venovenous hemodiafiltration (CVVHDF) and sustained low-efficiency dialysis filtration (SLED-f). We aimed at preventing KRT-related hypophosphatemia while optimizing acid-base equilibrium.
Methods
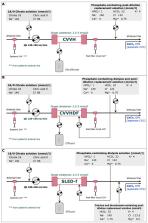
KRT was performed by the Prismax system (Baxter) and polyacrylonitrile AN69 filters (ST 150, 1.5 m 2, Baxter), combining a 18 mmol/L pre-dilution citrate solution (Regiocit 18/0, Baxter) with a phosphate-containing solution (HPO 4 2− 1.0 mmol/L, HCO 3 − 22.0 mmol/L; Biphozyl, Baxter). When needed, phosphate loss was replaced with sodium glycerophosphate pentahydrate (Glycophos™ 20 mmol/20 mL, Fresenius Kabi Norge AS, Halden, Norway). Serum citrate measurements were scheduled during each treatment. We analyzed data from three consecutive daily 8-h SLED-f sessions, as well as single 72-h CVVH or 72-h CVVHDF sessions. We used analysis of variance (ANOVA) for repeated measures to evaluate differences in variables means (i.e. serum phosphate, citrate). Because some patients received phosphate supplementation, we performed analysis of covariance (ANCOVA) for repeated measures modelling phosphate supplementation as a covariate.
Results
Forty-seven patients with acute kidney injury (AKI) or end stage kidney disease (ESKD) requiring KRT were included [11 CVVH, 11 CVVHDF and 25 SLED-f sessions; mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score 25 ± 7.0]. Interruptions for irreversible filter clotting were negligible. The overall incidence of hypophosphatemia (s-P levels <2.5 mg/dL) was 6.6%, and s-P levels were kept in the normality range irrespective of baseline values and the KRT modality. The acid-base balance was preserved, with no episode of citrate accumulation.
Graphical Abstract
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Intensity of renal support in critically ill patients with acute kidney injury.
- Record: found
- Abstract: found
- Article: not found
Intensity of continuous renal-replacement therapy in critically ill patients.
- Record: found
- Abstract: found
- Article: not found