- Record: found
- Abstract: found
- Article: found
Phenotype of NK-Like CD8(+) T Cells with Innate Features in Humans and Their Relevance in Cancer Diseases
Read this article at
Abstract
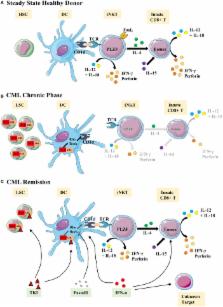
Unconventional T cells are defined by their capacity to respond to signals other than the well-known complex of peptides and major histocompatibility complex proteins. Among the burgeoning family of unconventional T cells, innate-like CD8(+) T cells in the mouse were discovered in the early 2000s. This subset of CD8(+) T cells bears a memory phenotype without having encountered a foreign antigen and can respond to innate-like IL-12 + IL-18 stimulation. Although the concept of innate memory CD8(+) T cells is now well established in mice, whether an equivalent memory NK-like T-cell population exists in humans remains under debate. We recently reported that CD8(+) T cells responding to innate-like IL-12 + IL-18 stimulation and co-expressing the transcription factor Eomesodermin (Eomes) and KIR/NKG2A membrane receptors with a memory/EMRA phenotype may represent a new, functionally distinct innate T cell subset in humans. In this review, after a summary on the known innate CD8(+) T-cell features in the mouse, we propose Eomes together with KIR/NKG2A and CD49d as a signature to standardize the identification of this innate CD8(+) T-cell subset in humans. Next, we discuss IL-4 and IL-15 involvement in the generation of innate CD8(+) T cells and particularly its possible dependency on the promyelocytic leukemia zinc-finger factor expressing iNKT cells, an innate T cell subset well documented for its susceptibility to tumor immune subversion. After that, focusing on cancer diseases, we provide new insights into the potential role of these innate CD8(+) T cells in a physiopathological context in humans. Based on empirical data obtained in cases of chronic myeloid leukemia, a myeloproliferative syndrome controlled by the immune system, and in solid tumors, we observe both the possible contribution of innate CD8(+) T cells to cancer disease control and their susceptibility to tumor immune subversion. Finally, we note that during tumor progression, innate CD8(+) T lymphocytes could be controlled by immune checkpoints. This study significantly contributes to understanding of the role of NK-like CD8(+) T cells and raises the question of the possible involvement of an iNKT/innate CD8(+) T cell axis in cancer.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Selective stimulation of T cell subsets with antibody-cytokine immune complexes.
- Record: found
- Abstract: found
- Article: not found
Naive T Cells Transiently Acquire a Memory-like Phenotype during Homeostasis-Driven Proliferation
- Record: found
- Abstract: found
- Article: not found