- Record: found
- Abstract: found
- Article: found
Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst
Read this article at
Abstract
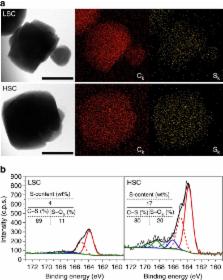
Maximum atom efficiency as well as distinct chemoselectivity is expected for electrocatalysis on atomically dispersed (or single site) metal centres, but its realization remains challenging so far, because carbon, as the most widely used electrocatalyst support, cannot effectively stabilize them. Here we report that a sulfur-doped zeolite-templated carbon, simultaneously exhibiting large sulfur content (17 wt% S), as well as a unique carbon structure (that is, highly curved three-dimensional networks of graphene nanoribbons), can stabilize a relatively high loading of platinum (5 wt%) in the form of highly dispersed species including site isolated atoms. In the oxygen reduction reaction, this catalyst does not follow a conventional four-electron pathway producing H 2O, but selectively produces H 2O 2 even over extended times without significant degradation of the activity. Thus, this approach constitutes a potentially promising route for producing important fine chemical H 2O 2, and also offers opportunities for tuning the selectivity of other electrochemical reactions on various metal catalysts.
Abstract
 Atomically dispersed metal catalysts display high atom efficiency for electrocatalytic
processes. Here, the authors report that sulfur-doped zeolite-templated carbon stabilizes
highly dispersed platinum species, predominantly as single-atom centres, and probe
its oxygen reduction selectivity.
Atomically dispersed metal catalysts display high atom efficiency for electrocatalytic
processes. Here, the authors report that sulfur-doped zeolite-templated carbon stabilizes
highly dispersed platinum species, predominantly as single-atom centres, and probe
its oxygen reduction selectivity.
Related collections
Most cited references25
- Record: found
- Abstract: not found
- Article: not found
Generalized Gradient Approximation Made Simple.
- Record: found
- Abstract: found
- Article: not found
Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts.
- Record: found
- Abstract: found
- Article: not found