- Record: found
- Abstract: found
- Article: found
Menstrual physiology: implications for endometrial pathology and beyond
Read this article at
Abstract
BACKGROUND
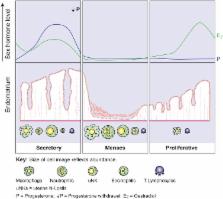
Each month the endometrium becomes inflamed, and the luminal portion is shed during menstruation. The subsequent repair is remarkable, allowing implantation to occur if fertilization takes place. Aberrations in menstrual physiology can lead to common gynaecological conditions, such as heavy or prolonged bleeding. Increased knowledge of the processes involved in menstrual physiology may also have translational benefits at other tissue sites.
METHODS
Pubmed and Cochrane databases were searched for all original and review articles published in English until April 2015. Search terms included ‘endometrium’, ‘menstruation’, ‘endometrial repair’, ‘endometrial regeneration’ ‘angiogenesis’, ‘inflammation’ and ‘heavy menstrual bleeding’ or ‘menorrhagia’.
RESULTS
Menstruation occurs naturally in very few species. Human menstruation is thought to occur as a consequence of preimplantation decidualization, conferring embryo selectivity and the ability to adapt to optimize function. We highlight how current and future study of endometrial inflammation, vascular changes and repair/regeneration will allow us to identify new therapeutic targets for common gynaecological disorders. In addition, we describe how increased knowledge of this endometrial physiology will have many translational applications at other tissue sites. We highlight the clinical applications of what we know, the key questions that remain and the scientific and medical possibilities for the future.
CONCLUSIONS
The study of menstruation, in both normal and abnormal scenarios, is essential for the production of novel, acceptable medical treatments for common gynaecological complaints. Furthermore, collaboration and communication with specialists in other fields could significantly advance the therapeutic potential of this dynamic tissue.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: found
VEGF as a Key Mediator of Angiogenesis in Cancer
- Record: found
- Abstract: found
- Article: not found
Natural killer cells and pregnancy.
- Record: found
- Abstract: found
- Article: not found