- Record: found
- Abstract: found
- Article: found
Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation

Read this article at
Abstract
Aims
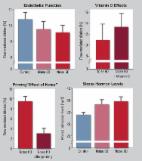
Aircraft noise causes endothelial dysfunction, oxidative stress, and inflammation. Transportation noise increases the incidence of coronary artery disease, hypertension, and stroke. The underlying mechanisms are not well understood. Herein, we investigated effects of phagocyte-type NADPH oxidase ( Nox2) knockout and different noise protocols (around-the-clock, sleep/awake phase noise) on vascular and cerebral complications in mice.
Methods and results
C57BL/6j and Nox2 −/− ( gp91phox −/−) mice were exposed to aircraft noise (maximum sound level of 85 dB(A), average sound pressure level of 72 dB(A)) around-the-clock or during sleep/awake phases for 1, 2, and 4 days. Adverse effects of around-the-clock noise on the vasculature and brain were mostly prevented by Nox2 deficiency. Around-the-clock aircraft noise of the mice caused the most pronounced vascular effects and dysregulation of Foxo3/circadian clock as revealed by next generation sequencing (NGS), suggesting impaired sleep quality in exposed mice. Accordingly, sleep but not awake phase noise caused increased blood pressure, endothelial dysfunction, increased markers of vascular/systemic oxidative stress, and inflammation. Noise also caused cerebral oxidative stress and inflammation, endothelial and neuronal nitric oxide synthase (e/nNOS) uncoupling, nNOS mRNA and protein down-regulation, and Nox2 activation. NGS revealed similarities in adverse gene regulation between around-the-clock and sleep phase noise. In patients with established coronary artery disease, night-time aircraft noise increased oxidative stress, and inflammation biomarkers in serum.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies.
- Record: found
- Abstract: found
- Article: not found
Myocardial infarction accelerates atherosclerosis
- Record: found
- Abstract: found
- Article: found