- Record: found
- Abstract: found
- Article: found
High-Throughput Toxicity and Phenotypic Screening of 3D Human Neural Progenitor Cell Cultures on a Microarray Chip Platform
Read this article at
Summary
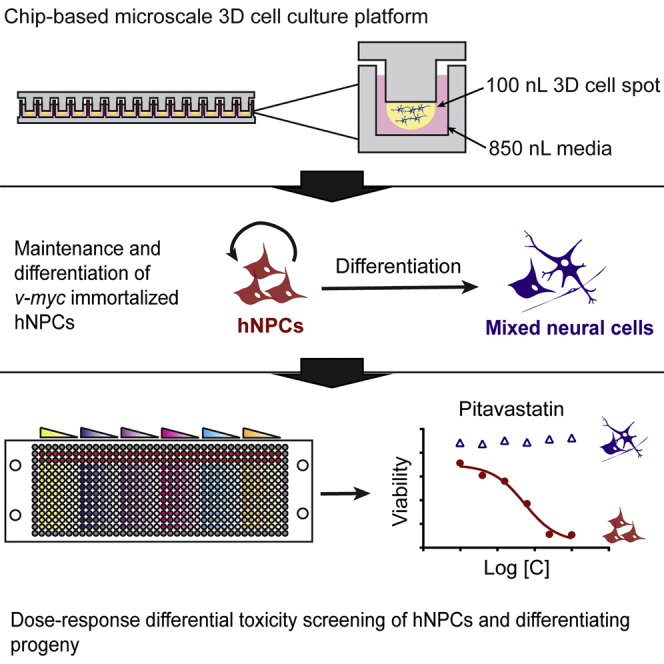
A 3D cell culture chip was used for high-throughput screening of a human neural progenitor cell line. The differential toxicity of 24 compounds was determined on undifferentiated and differentiating NPCs. Five compounds led to significant differences in IC 50 values between undifferentiated and differentiating cultures. This platform has potential use in phenotypic screening to elucidate molecular toxicology on human stem cells.
Graphical Abstract
Highlights
-
•
Demonstrated chip platform for HTS of protein expression and toxicity of 3D cultures
-
•
Dose-response viability and proliferation of a 24-compound library on human NPC lines
-
•
Assessed differential toxicity between progenitors and differentiating progeny
-
•
Identified five compounds more toxic to undifferentiated progenitors
Abstract
Dordick and colleagues demonstrate a 3D cell culture chip for high-throughput screening of immortalized human neural progenitor cells. The dose-response toxicity of 24 compounds was determined on undifferentiated and differentiating hNPCs. Five compounds led to significant differences in IC 50 values between undifferentiated and differentiating cultures. This platform has potential use in phenotypic screening to elucidate molecular toxicology on human stem cells.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
In vitro differentiation of transplantable neural precursors from human embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found
Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis.
- Record: found
- Abstract: found
- Article: not found