- Record: found
- Abstract: found
- Article: found
The search for blood biomarkers that indicate risk of adverse neurodevelopmental outcomes in fetal growth restriction
Read this article at
Abstract
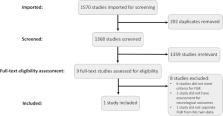
Fetal growth restriction (FGR) impacts 5%–10% of pregnancies and is associated with increased risk of mortality and morbidity. Although adverse neurodevelopmental outcomes are observed in up to 50% of FGR infants, a diagnosis of FGR does not indicate the level of risk for an individual infant and these infants are not routinely followed up to assess neurodevelopmental outcomes. Identifying FGR infants at increased risk of adverse neurodevelopmental outcomes would greatly assist in providing appropriate support and interventions earlier, resulting in improved outcomes. However, current methods to detect brain injury around the time of birth lack the sensitivity required to detect the more subtle alterations associated with FGR. Blood biomarkers have this potential. This systematic review assessed the current literature on blood biomarkers for identifying FGR infants at increased risk of adverse neurodevelopmental outcomes at >12 months after birth. Four databases were searched from inception to 22 February 2024. Articles were assessed for meeting the inclusion criteria by two reviewers. The quality of the included article was assessed using Quality Assessment of Diagnostic Accuracy Studies-2. A summary of findings is presented as insufficient articles were identified for meta-analysis. Excluding duplicates, 1,368 records were screened with only 9 articles considered for full text review. Only one article met all the inclusion criteria. Quality assessment indicated low risk of bias. Both blood biomarkers investigated in this study, neuron specific enolase and S100B, demonstrated inverse relationships with neurodevelopmental assessments at 2 years. Four studies did not meet all the inclusion criteria yet identified promising findings for metabolites and cytokines which are discussed here. These findings support the need for further research and highlight the potential for blood biomarkers to predict adverse outcomes.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=369242, Identifier CRD42022369242.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies.
- Record: found
- Abstract: found
- Article: not found
Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment.
- Record: found
- Abstract: found
- Article: not found