- Record: found
- Abstract: found
- Article: not found
Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities
Abstract
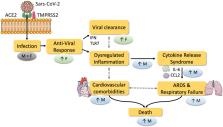
The high mortality rate of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection is a critical concern of the coronavirus disease 2019 (COVID-19) pandemic. Strikingly, men account for the majority of COVID-19 deaths, with current figures ranging from 59% to 75% of total mortality. However, despite clear implications in relation to COVID-19 mortality, most research has not considered sex as a critical factor in data analysis. Here, we highlight fundamental biological differences that exist between males and females, and how these may make significant contributions to the male-biased COVID-19 mortality. We present preclinical evidence identifying the influence of biological sex on the expression and regulation of angiotensin-converting enzyme 2 (ACE2), which is the main receptor used by SARS-CoV-2 to enter cells. However, we note that there is a lack of reports showing that sexual dimorphism of ACE2 expression exists and is of functional relevance in humans. In contrast, there is strong evidence, especially in the context of viral infections, that sexual dimorphism plays a central role in the genetic and hormonal regulation of immune responses, both of the innate and the adaptive immune system. We review evidence supporting that ineffective anti-SARS-CoV-2 responses, coupled with a predisposition for inappropriate hyperinflammatory responses, could provide a biological explanation for the male bias in COVID-19 mortality. A prominent finding in COVID-19 is the increased risk of death with pre-existing cardiovascular comorbidities, such as hypertension, obesity, and age. We contextualize how important features of sexual dimorphism and inflammation in COVID-19 may exhibit a reciprocal relationship with comorbidities, and explain their increased mortality risk. Ultimately, we demonstrate that biological sex is a fundamental variable of critical relevance to our mechanistic understanding of SARS-CoV-2 infection and the pursuit of effective COVID-19 preventative and therapeutic strategies.
Related collections
Most cited references147
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Record: found
- Abstract: found
- Article: not found
Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
