- Record: found
- Abstract: found
- Article: found
Thapsigargin—From Traditional Medicine to Anticancer Drug
Read this article at
Abstract
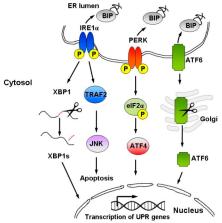
A sesquiterpene lactone, thapsigargin, is a phytochemical found in the roots and fruits of Mediterranean plants from Thapsia L. species that have been used for centuries in folk medicine to treat rheumatic pain, lung diseases, and female infertility. More recently thapsigargin was found to be a potent cytotoxin that induces apoptosis by inhibiting the sarcoplasmic/endoplasmic reticulum Ca 2+ ATPase (SERCA) pump, which is necessary for cellular viability. This biological activity encouraged studies on the use of thapsigargin as a novel antineoplastic agent, which were, however, hampered due to high toxicity of this compound to normal cells. In this review, we summarized the recent knowledge on the biological activity and molecular mechanisms of thapsigargin action and advances in the synthesis of less-toxic thapsigargin derivatives that are being developed as novel anticancer drugs.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
The Unfolded Protein Response and Cell Fate Control.
- Record: found
- Abstract: found
- Article: not found
The role of endoplasmic reticulum stress in human pathology.
- Record: found
- Abstract: found
- Article: not found