- Record: found
- Abstract: found
- Article: found
Plasticity in the Hippocampus, Neurogenesis and Drugs of Abuse
Read this article at
Abstract
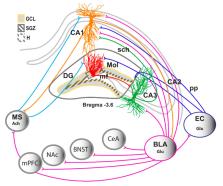
Synaptic plasticity in the hippocampus assists with consolidation and storage of long-lasting memories. Decades of research has provided substantial information on the cellular and molecular mechanisms underlying synaptic plasticity in the hippocampus, and this review discusses these mechanisms in brief. Addiction is a chronic relapsing disorder with loss of control over drug taking and drug seeking that is caused by long-lasting memories of drug experience. Relapse to drug use is caused by exposure to context and cues associated with the drug experience, and is a major clinical problem that contributes to the persistence of addiction. This review also briefly discusses some evidence that drugs of abuse alter plasticity in the hippocampus, and that development of novel treatment strategies that reverse or prevent drug-induced synaptic alterations in the hippocampus may reduce relapse behaviors associated with addiction.
Related collections
Most cited references194
- Record: found
- Abstract: found
- Article: not found
Short-term synaptic plasticity.
- Record: found
- Abstract: found
- Article: not found
Mechanisms and functional implications of adult neurogenesis.
- Record: found
- Abstract: found
- Article: not found