- Record: found
- Abstract: found
- Article: found
Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue From Prepubertal Boys
Read this article at
Abstract
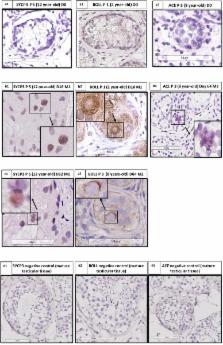
While in mice various studies have described the completion of spermatogenesis in vitro using either organotypic culture of prepubertal testicular tissue or 3D culture of isolated cells, in humans it has not been possible to achieve germ cell differentiation from immature testicular tissue (ITT). In our study, we evaluated the ability of human ITT to differentiate via a long-term organotypic culture of frozen–thawed 1 mm 3 testicular fragments from five prepubertal boys in two different culture media. Tissue and supernatants were analyzed at regular intervals up to day 139. Sertoli cell (SC) viability and maturation was evaluated using immunohistochemistry (IHC) for SOX9, GDNF, anti-Mullerian hormone (AMH) and androgen receptor (AR), and AMH concentration in supernatants. Spermatogonia (SG) and proliferating cells were identified by MAGE-A4 (for SG) and Ki67 (for proliferating cells) via immunohistochemistry (IHC). Apoptotic cells were studied by active caspase 3. To evaluate Leydig cell (LC) functionality testosterone was measured in the supernatants and steroidogenic acute regulatory protein (STAR) IHC was performed. Germ cell differentiation was evaluated on Hematoxylin-Eosin histological sections, via IHC for synaptonemal complex 3 (SYCP3) for spermatocytes, Protein boule-like (BOLL) for spermatocytes and round spermatids, angiotensin-converting enzyme (ACE), protamine 2 and transition protein 1 (for elongated spermatids) and via chromogenic in situ hybridization (CISH). We reported the generation of meiotic and postmeiotic cells after 16 days of culture, as shown by the histological analyses, the presence of differentiation markers and the increase of haploid germ cells. We showed SC viability and maturation by a decrease of AMH secretion in the supernatants ( p ≤ 0.001) while the number of SOX9 positive cells did not show any variation. A decrease of spermatogonia ( p ≤ 0.001) was observed. The number of apoptotic cells did not vary. LC functionality was shown by the increase in STAR expression ( p ≤ 0.007) and a peak in testosterone secretion, followed by a reduction ( p ≤ 0.001) with stabilization. According to our knowledge, this is the first report of generation of haploid cells in human ITT. Differentiating germ cells have to be further evaluated for their ability to complete differentiation, their fecundability and epigenetic characteristics.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
In vitro production of functional sperm in cultured neonatal mouse testes.
- Record: found
- Abstract: found
- Article: not found
A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys.
- Record: found
- Abstract: found
- Article: not found