- Record: found
- Abstract: found
- Article: not found
Rank Signaling Links the Development of Invariant γδ T Cell Progenitors and Aire + Medullary Epithelium

Read this article at
Summary
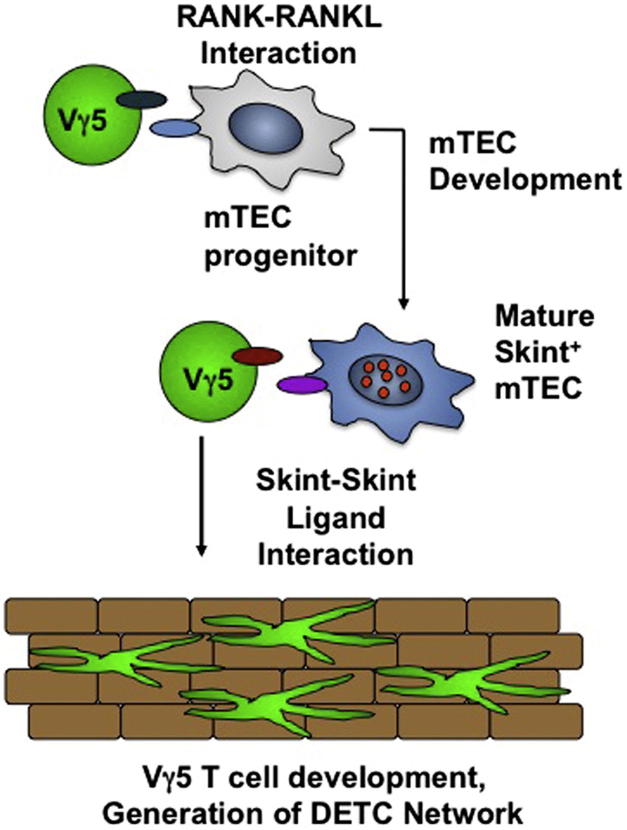
The thymic medulla provides a specialized microenvironment for the negative selection of T cells, with the presence of autoimmune regulator (Aire)-expressing medullary thymic epithelial cells (mTECs) during the embryonic-neonatal period being both necessary and sufficient to establish long-lasting tolerance. Here we showed that emergence of the first cohorts of Aire + mTECs at this key developmental stage, prior to αβ T cell repertoire selection, was jointly directed by Rankl + lymphoid tissue inducer cells and invariant Vγ5 + dendritic epidermal T cell (DETC) progenitors that are the first thymocytes to express the products of gene rearrangement. In turn, generation of Aire + mTECs then fostered Skint-1-dependent, but Aire-independent, DETC progenitor maturation and the emergence of an invariant DETC repertoire. Hence, our data attributed a functional importance to the temporal development of Vγ5 + γδ T cells during thymus medulla formation for αβ T cell tolerance induction and demonstrated a Rank-mediated reciprocal link between DETC and Aire + mTEC maturation.
Abstract
Highlights
► Invariant Vγ5 + thymocytes regulate formation of Aire + medullary thymic epithelium ► Generation of an invariant Vγ5 + T cell population requires thymus medulla development ► Skint-1-mediated Vγ5 + thymocyte development is Aire independent ► Dependency on Tnfrsf11a links γδ T cell and medullary epithelium development
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Projection of an immunological self shadow within the thymus by the aire protein.
- Record: found
- Abstract: found
- Article: not found
Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self.
- Record: found
- Abstract: found
- Article: not found