- Record: found
- Abstract: found
- Article: found
Carbon mineralization pathways for carbon capture, storage and utilization
brief-report
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
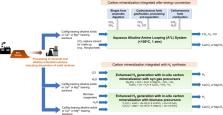
Carbon mineralization is a versatile and thermodynamically downhill process that can be harnessed for capturing, storing, and utilizing CO 2 to synthesize products with enhanced properties. Here the author discusses the advances in and challenges of carbon mineralization, and concludes that tuning the chemical interactions involved will allow us to unlock its potential for advancing low carbon energy and resource conversion pathways.
Related collections
Most cited references29
- Record: found
- Abstract: not found
- Article: not found
Water-gas shift reaction: finding the mechanistic boundary
C. RHODES, G.J. Hutchings, A.M. Ward (1995)