- Record: found
- Abstract: found
- Article: found
The molecular machinery of regulated cell death
Read this article at
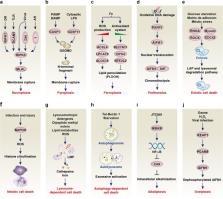
Abstract
Cells may die from accidental cell death (ACD) or regulated cell death (RCD). ACD is a biologically uncontrolled process, whereas RCD involves tightly structured signaling cascades and molecularly defined effector mechanisms. A growing number of novel non-apoptotic forms of RCD have been identified and are increasingly being implicated in various human pathologies. Here, we critically review the current state of the art regarding non-apoptotic types of RCD, including necroptosis, pyroptosis, ferroptosis, entotic cell death, netotic cell death, parthanatos, lysosome-dependent cell death, autophagy-dependent cell death, alkaliptosis and oxeiptosis. The in-depth comprehension of each of these lethal subroutines and their intercellular consequences may uncover novel therapeutic targets for the avoidance of pathogenic cell loss.
Related collections
Most cited references175
- Record: found
- Abstract: found
- Article: not found
ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition.
- Record: found
- Abstract: not found
- Article: not found