- Record: found
- Abstract: found
- Article: found
Drug delivery system for the extended-release of larotrectinib based on a biocompatible Fe-based metal-organic framework: synthesis, characterization, in vitro release properties and antitumor evaluation
Read this article at
Abstract
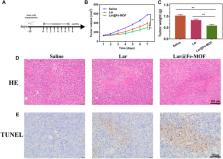
Larotrectinib (Lar) is an orally administered tropomyosin receptor kinase (Trk) inhibitor with broad-spectrum antitumor activity that is available in clinical dosage forms as capsules and oral solutions. Currently, corresponding research is focused on developing new extended-release formulation systems for Lar. In this study, a biocompatible Fe-based metal-organic framework (Fe-MOF) carrier was synthesized by a solvent-based method, and a sustained-release drug delivery system (Lar@Fe-MOF) was constructed by nanoprecipitation and Lar loading. Lar@Fe-MOF was characterized by transmission electron microscopy (TEM), differential scanning calorimetry (DSC), fourier transform infrared (FTIR) spectroscopy, and thermogravimetric analysis (TGA), and its drug loading capacity and drug release properties were measured by ultraviolet–visible (UV–vis) spectroscopy. Then, the toxicity and biocompatibility of the Fe-MOF carriers were evaluated using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and hemocompatibility assays. Finally, the anticancer potential of Lar@Fe-MOF was investigated. The TEM results showed that Lar@Fe-MOF had a homogeneous fusiform nanostructural morphology. The DSC and FTIR results showed that Fe-MOF carriers were successfully synthesized and loaded with Lar, which was mainly in an amorphous form. Lar@Fe-MOF showed a large drug loading capacity (–10%) and significant slow-release properties in vitro. The MTT assay results showed that Lar@Fe-MOF had good dose-dependent anticancer activity. The in vivo pharmacodynamic assay results showed that Fe-MOF significantly increased the anticancer activity of Lar and was biocompatible. In conclusion, the Lar@Fe-MOF system developed in this study is a promising drug delivery platform because it is easy to manufacture, has high biocompatibility and ideal drug release and accumulation, can effectively eliminate tumors with improved safety and is expected to further expand therapeutic applications.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Polymer microspheres for controlled drug release.

- Record: found
- Abstract: found
- Article: found
Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications
- Record: found
- Abstract: found
- Article: not found