- Record: found
- Abstract: found
- Article: found
Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking
Read this article at
Abstract
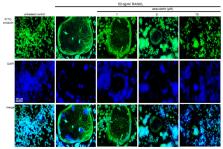
For the optimal resorption of mineralized bone matrix, osteoclasts require the generation of the ruffled border and acidic resorption lacuna through lysosomal trafficking and exocytosis. Coumarin-type aesculetin is a naturally occurring compound with anti-inflammatory and antibacterial effects. However, the direct effects of aesculetin on osteoclastogenesis remain to be elucidated. This study found that aesculetin inhibited osteoclast activation and bone resorption through blocking formation and exocytosis of lysosomes. Raw 264.7 cells were differentiated in the presence of 50 ng/mL receptor activator of nuclear factor-κB ligand (RANKL) and treated with 1–10 μM aesculetin. Differentiation, bone resorption, and lysosome biogenesis of osteoclasts were determined by tartrate-resistance acid phosphatase (TRAP) staining, bone resorption assay, Western blotting, immunocytochemical analysis, and LysoTracker staining. Aesculetin inhibited RANKL-induced formation of multinucleated osteoclasts with a reduction of TRAP activity. Micromolar aesculetin deterred the actin ring formation through inhibition of induction of αvβ3 integrin and Cdc42 but not cluster of differentiation 44 (CD44) in RANKL-exposed osteoclasts. Administering aesculetin to RANKL-exposed osteoclasts attenuated the induction of autophagy-related proteins, microtubule-associated protein light chain 3, and small GTPase Rab7, hampering the lysosomal trafficking onto ruffled border crucial for bone resorption. In addition, aesculetin curtailed cellular induction of Pleckstrin homology domain-containing protein family member 1 and lissencephaly-1 involved in lysosome positioning to microtubules involved in the lysosomal transport within mature osteoclasts. These results demonstrate that aesculetin retarded osteoclast differentiation and impaired lysosomal trafficking and exocytosis for the formation of the putative ruffled border. Therefore, aesculetin may be a potential osteoprotective agent targeting RANKL-induced osteoclastic born resorption for medicinal use.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Osteoclast differentiation and activation.
- Record: found
- Abstract: found
- Article: not found