- Record: found
- Abstract: found
- Article: found
Roles of focal adhesion proteins in skeleton and diseases
Read this article at
Abstract
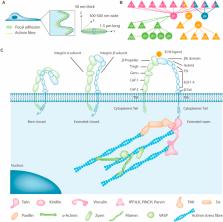
The skeletal system, which contains bones, joints, tendons, ligaments and other elements, plays a wide variety of roles in body shaping, support and movement, protection of internal organs, production of blood cells and regulation of calcium and phosphate metabolism. The prevalence of skeletal diseases and disorders, such as osteoporosis and bone fracture, osteoarthritis, rheumatoid arthritis, and intervertebral disc degeneration, increases with age, causing pain and loss of mobility and creating a huge social and economic burden globally. Focal adhesions (FAs) are macromolecular assemblies that are composed of the extracellular matrix (ECM), integrins, intracellular cytoskeleton and other proteins, including kindlin, talin, vinculin, paxillin, pinch, Src, focal adhesion kinase (FAK) and integrin-linked protein kinase (ILK) and other proteins. FA acts as a mechanical linkage connecting the ECM and cytoskeleton and plays a key role in mediating cell–environment communications and modulates important processes, such as cell attachment, spreading, migration, differentiation and mechanotransduction, in different cells in skeletal system by impacting distinct outside-in and inside-out signaling pathways. This review aims to integrate the up-to-date knowledge of the roles of FA proteins in the health and disease of skeletal system and focuses on the specific molecular mechanisms and underlying therapeutic targets for skeletal diseases.
Graphical abstract
Related collections
Most cited references159
- Record: found
- Abstract: not found
- Article: not found
Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review

- Record: found
- Abstract: found
- Article: found
