- Record: found
- Abstract: found
- Article: found
Use of constitutive and inducible oncogene-containing iPSCs as surrogates for transgenic mice to study breast oncogenesis
Read this article at
Abstract
Background
Powerful constitutive and inducible transgenic / bitransgenic / tritransgenic murine models of breast cancer have been used over the past two decades to shed light on the molecular mechanisms by which the given transgenic oncogenes have interacted with other cellular genes and set in motion breast cancer initiation and progression. However, these transgenic models, as in vivo models only, are expensive and restrictive in the opportunities they provide to manipulate the experimental variables that would enable a better understanding of the molecular events related to initial transformation and the target cell being transformed.
Methods
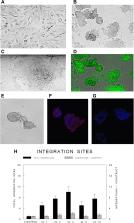
To overcome some of these limitations, we derived oncogene-containing induced pluripotent stem cell (iPSC) clones from tail vein fibroblasts of these transgenic mice and manipulated them both in vitro and in vivo in non-transgenic background mice. We created the iPSC clones with a relatively low M.O.I, producing retroviral integrations which averaged only 1 to 2 sites per retroviral plasmid construct used.
Results
Most iPSC clones derived from each group displayed an essentially normal murine karyotype, strong expression of the exogenous reprogrammable genes and significant expression of characteristic endogenous murine surface stem cell markers including SSEA-1 (CD15), PECAM-1 (CD31), Ep-Cam (CD326), and Nectin (CD112), but no expression of their transgene. A majority (75%) of iPSC clones displayed a normal murine karyotype but 25% exhibited a genomically unstable karyotype. However, even these later clones exhibited stable and characteristic iPSC properties. When injected orthotopically, select iPSC clones, either constitutive or inducible, no longer expressed their exogenous pluripotency reprogramming factors but expressed their oncogenic transgene (PyVT or ErbB2) and participated in both breast ontogenesis and subsequent oncogenesis. When injected non-orthotopically or when differentiated in vitro along several different non-mammary lineages of differentiation, the iPSC clones failed to do so. Although many clones developed anticipated teratomas, select iPSC clones under the appropriate constitutive or inducible conditions exhibited both breast ontogenesis and oncogenesis through the same stages as exhibited by their transgenic murine parents and, as such, represent transgenic surrogates.
Conclusions
The iPSC clones offer a number of advantages over transgenic mice including cost, the ability to manipulate and tag in vitro, and create an in vitro model of breast ontogeny and oncogenesis that can be used to gain additional insights into the differentiated status of the target cell which is susceptible to transformation. In addition, the use of these oncogene-containing iPSC clones can be used in chemopreventive studies of breast cancer.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2019
- Record: found
- Abstract: found
- Article: found
Aging, Cellular Senescence, and Cancer
- Record: found
- Abstract: found
- Article: not found