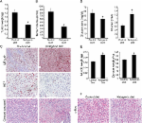
Background Aberrant energy metabolism is a hallmark of cancer. To fulfill the increased energy requirements, tumor cells secrete cytokines/factors inducing muscle and fat degradation in cancer patients, a condition known as cancer cachexia. It accounts for nearly 20% of all cancer-related deaths. However, the mechanistic basis of cancer cachexia and therapies targeting cancer cachexia thus far remain elusive. A ketogenic diet, a high-fat and low-carbohydrate diet that elevates circulating levels of ketone bodies (i.e., acetoacetate, β-hydroxybutyrate, and acetone), serves as an alternative energy source. It has also been proposed that a ketogenic diet leads to systemic metabolic changes. Keeping in view the significant role of metabolic alterations in cancer, we hypothesized that a ketogenic diet may diminish glycolytic flux in tumor cells to alleviate cachexia syndrome and, hence, may provide an efficient therapeutic strategy. Results We observed reduced glycolytic flux in tumor cells upon treatment with ketone bodies. Ketone bodies also diminished glutamine uptake, overall ATP content, and survival in multiple pancreatic cancer cell lines, while inducing apoptosis. A decrease in levels of c-Myc, a metabolic master regulator, and its recruitment on glycolytic gene promoters, was in part responsible for the metabolic phenotype in tumor cells. Ketone body-induced intracellular metabolomic reprogramming in pancreatic cancer cells also leads to a significantly diminished cachexia in cell line models. Our mouse orthotopic xenograft models further confirmed the effect of a ketogenic diet in diminishing tumor growth and cachexia. Conclusions Thus, our studies demonstrate that the cachectic phenotype is in part due to metabolic alterations in tumor cells, which can be reverted by a ketogenic diet, causing reduced tumor growth and inhibition of muscle and body weight loss.