- Record: found
- Abstract: found
- Article: found
ENaC in Salt-Sensitive Hypertension: Kidney and Beyond
Read this article at
Abstract
Purpose of Review
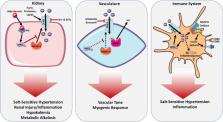
The main goal of this article is to discuss the role of the epithelial sodium channel (ENaC) in extracellular fluid and blood pressure regulation.
Recent Findings
Besides its role in sodium handling in the kidney, recent studies have found that ENaC expressed in other cells including immune cells can influence blood pressure via extra-renal mechanisms. Dendritic cells (DCs) are activated and contribute to salt-sensitive hypertension in an ENaC-dependent manner. We discuss recent studies on how ENaC is regulated in both the kidney and other sites including the vascular smooth muscles, endothelial cells, and immune cells. We also discuss how this extra-renal ENaC can play a role in salt-sensitive hypertension and its promise as a novel therapeutic target.
Summary
The role of ENaC in blood pressure regulation in the kidney has been well studied. Recent human gene sequencing efforts have identified thousands of variants among the genes encoding ENaC, and research efforts to determine if these variants and their expression in extra-renal tissue play a role in hypertension will advance our understanding of the pathogenesis of ENaC-mediated cardiovascular disease and lead to novel therapeutic targets.
Related collections
Most cited references113
- Record: found
- Abstract: found
- Article: not found
Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits.
- Record: found
- Abstract: found
- Article: not found