- Record: found
- Abstract: found
- Article: found
TGF‐β signaling directly regulates transcription and functional expression of the electrogenic sodium bicarbonate cotransporter 1, NBCe1 (SLC4A4), via Smad4 in mouse astrocytes
Read this article at
Abstract
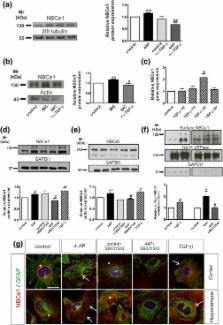
The electrogenic sodium bicarbonate cotransporter NBCe1 (SLC4A4) expressed in astrocytes regulates intracellular and extracellular pH. Here, we introduce transforming growth factor beta (TGF‐β) as a novel regulator of NBCe1 transcription and functional expression. Using hippocampal slices and primary hippocampal and cortical astrocyte cultures, we investigated regulation of NBCe1 and elucidated the underlying signaling pathways by RT‐PCR, immunoblotting, immunofluorescence, intracellular H( +) recording using the H( +) ‐sensitive dye 2′,7′‐bis‐(carboxyethyl)‐5‐(and‐6)‐carboxyfluorescein, mink lung epithelial cell (MLEC) assay, and chromatin immunoprecipitation. Activation of TGF‐β signaling significantly upregulated transcript, protein, and surface expression of NBCe1. These effects were TGF‐β receptor‐mediated and suppressed following inhibition of JNK and Smad signaling. Moreover, 4‐aminopyridine (4AP)‐dependent NBCe1 regulation requires TGF‐β. TGF‐β increased the rate and amplitude of intracellular H + changes upon challenging NBCe1 in wild‐type astrocytes but not in cortical astrocytes from Slc4a4‐deficient mice. A Smad4 binding sequence was identified in the NBCe1 promoter and Smad4 binding increased after activation of TGF‐β signaling. The data show for the first time that NBCe1 is a direct target of TGF‐β/Smad4 signaling. Through activation of the canonical pathway TGF‐β acts directly on NBCe1 by binding of Smad4 to the NBCe1 promoter and regulating its transcription, followed by increased protein expression and transport activity.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Astrocyte control of synaptic transmission and neurovascular coupling.
- Record: found
- Abstract: found
- Article: not found
Extracellular control of TGFbeta signalling in vascular development and disease.
- Record: found
- Abstract: found
- Article: not found