- Record: found
- Abstract: found
- Article: found
Timeliness of national notifiable diseases surveillance system in Korea: a cross-sectional study
Read this article at
Abstract
Background
With the increase of international travels, infectious disease control is gaining a greater importance across regional borders. Adequate surveillance system function is crucial to prevent a global spread of infectious disease at the earliest stage. There have been limited reports on the characteristics of infectious disease surveillance in Asia. The authors studied the timeliness of the Korean National Notifiable Disease Surveillance System with regard to major notifiable diseases from 2001 to 2006.
Methods
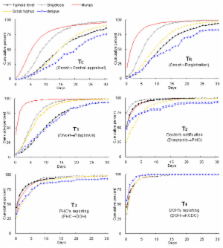
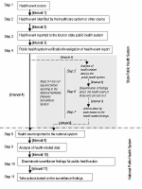
Six notifiable infectious diseases reported relatively frequently were included in this study. Five diseases were selected by the criteria of reported cases > 100 per year: typhoid fever, shigellosis, mumps, scrub typhus, and hemorrhagic fever with renal syndrome. In addition, dengue fever was also included to represent an emerging disease, despite its low number of cases. The diseases were compared for the proportion notified within the recommended time limits, median time lags, and for the cumulative distribution of time lags at each surveillance step between symptom onset and date of notification to the Korea Centers for Disease Control and Prevention (KCDC).
Results
The proportion of cases reported in time was lower for disease groups with a recommended time limit of 1 day compared with 7 days (60%–70% vs. > 80%). The median time from disease onset to notification to KCDC ranged between 6 and 20 days. The median time from onset to registration at the local level ranged between 2 and 15 days. Distribution of time lags showed that main delays arose in the time from onset to diagnosis. There were variations in timeliness by disease categories and surveillance steps.
Conclusion
Time from disease onset to diagnosis generally contributed most to the delay in reporting. It is needed to promote public education and to improve clinical guidelines. Rapid reporting by doctors should be encouraged, and unification of recommended reporting time limit can be helpful. Our study also demonstrates the utility of the overall assessment of time-lag distributions for disease-specific strategies to improve surveillance.
Related collections
Most cited references15
- Record: found
- Abstract: not found
- Article: not found
Completeness of notifiable infectious disease reporting in the United States: an analytical literature review.
- Record: found
- Abstract: found
- Article: found
Evaluation of reporting timeliness of public health surveillance systems for infectious diseases

- Record: found
- Abstract: found
- Article: found