- Record: found
- Abstract: found
- Article: found
Astrocytes convert network excitation to tonic inhibition of neurons
Read this article at
Abstract
Background
Glutamate and γ-aminobutyric acid (GABA) transporters play important roles in balancing excitatory and inhibitory signals in the brain. Increasing evidence suggest that they may act concertedly to regulate extracellular levels of the neurotransmitters.
Results
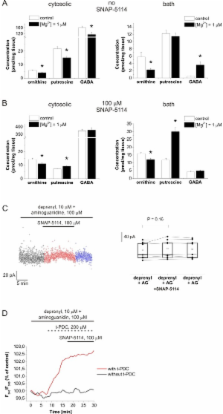
Here we present evidence that glutamate uptake-induced release of GABA from astrocytes has a direct impact on the excitability of pyramidal neurons in the hippocampus. We demonstrate that GABA, synthesized from the polyamine putrescine, is released from astrocytes by the reverse action of glial GABA transporter (GAT) subtypes GAT-2 or GAT-3. GABA release can be prevented by blocking glutamate uptake with the non-transportable inhibitor DHK, confirming that it is the glutamate transporter activity that triggers the reversal of GABA transporters, conceivably by elevating the intracellular Na + concentration in astrocytes. The released GABA significantly contributes to the tonic inhibition of neurons in a network activity-dependent manner. Blockade of the Glu/GABA exchange mechanism increases the duration of seizure-like events in the low-[Mg 2+] in vitro model of epilepsy. Under in vivo conditions the increased GABA release modulates the power of gamma range oscillation in the CA1 region, suggesting that the Glu/GABA exchange mechanism is also functioning in the intact hippocampus under physiological conditions.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Astrocyte control of synaptic transmission and neurovascular coupling.
- Record: found
- Abstract: found
- Article: not found