- Record: found
- Abstract: found
- Article: found
Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial
Read this article at
Abstract
Objective
To show that limiting dual antiplatelet therapy (DAPT) to six months in patients with event-free ST-elevation myocardial infarction (STEMI) results in a non-inferior clinical outcome versus DAPT for 12 months.
Setting
Patients with STEMI treated with primary percutaneous coronary intervention (PCI) and second generation zotarolimus-eluting stent.
Participants
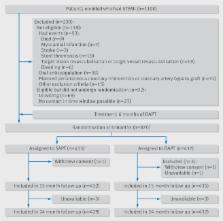
Patients with STEMI aged 18 to 85 that underwent a primary PCI with the implantation of second generation drug-eluting stents were enrolled in the trial. Patients that were event-free at six months after primary PCI were randomised at this time point.
Interventions
Patients that were taking DAPT and were event-free at six months were randomised 1:1 to single antiplatelet therapy (SAPT) (ie, aspirin only) or to DAPT for an additional six months. All patients that were randomised were then followed for another 18 months (ie, 24 months after the primary PCI).
Main outcome measures
The primary endpoint was a composite of all cause mortality, any myocardial infarction, any revascularisation, stroke, and thrombolysis in myocardial infarction major bleeding at 18 months after randomisation.
Results
A total of 1100 patients were enrolled in the trial between 19 December 2011 and 30 June 2015. 870 were randomised: 432 to SAPT versus 438 to DAPT. The primary endpoint occurred in 4.8% of patients receiving SAPT versus 6.6% of patients receiving DAPT (hazard ratio 0.73, 95% confidence interval 0.41 to 1.27, P=0.26). Non-inferiority was met (P=0.004 for non-inferiority), as the upper 95% confidence interval of 1.27 was smaller than the prespecified non-inferiority margin of 1.66.
Related collections
Most cited references32
- Record: found
- Abstract: not found
- Article: not found
2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions.
- Record: found
- Abstract: found
- Article: not found
Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials
- Record: found
- Abstract: found
- Article: not found