- Record: found
- Abstract: found
- Article: found
Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice
Read this article at
Abstract
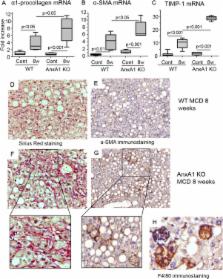
Annexin A1 (AnxA1) is an effector of the resolution of inflammation and is highly effective in terminating acute inflammatory responses. However, its role in chronic settings is less investigated. Because changes in AnxA1 expression within adipose tissue characterize obesity in mice and humans, we queried a possible role for AnxA1 in the pathogenesis of nonalcoholic steatohepatitis (NASH), a disease commonly associated with obesity. NASH was induced in wild-type (WT) and AnxA1 knockout (AnxA1 KO) C57BL/6 mice by feeding a methionine-choline deficient (MCD) diet up to 8 weeks. In MCD-fed WT mice, hepatic AnxA1 increased in parallel with progression of liver injury. This mediator was also detected in liver biopsies from patients with NASH and its degree of expression inversely correlated with the extent of fibrosis. In both humans and rodents, AnxA1 production was selectively localized in liver macrophages. NASH in AnxA1 KO mice was characterized by enhanced lobular inflammation resulting from increased macrophage recruitment and exacerbation of the M1 phenotype. Consistently, in vitro addition of recombinant AnxA1 to macrophages isolated from NASH livers down-modulated M1 polarization through stimulation of interleukin-10 production. Furthermore, the degree of hepatic fibrosis was enhanced in MCD-fed AnxA1 KO mice, an effect associated with augmented liver production of the profibrotic lectin, galectin-3. Accordingly, AnxA1 addition to isolated hepatic macrophages reduced galectin-3 expression. Conclusions: Macrophage-derived AnxA1 plays a functional role in modulating hepatic inflammation and fibrogenesis during NASH progression, suggesting the possible use of AnxA1 analogs for therapeutic control of this disease.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Annexin A1 and glucocorticoids as effectors of the resolution of inflammation.

- Record: found
- Abstract: found
- Article: not found