- Record: found
- Abstract: found
- Article: found
Does Inflammation Play a Major Role in the Pathogenesis of Alzheimer's Disease?

Read this article at
Abstract
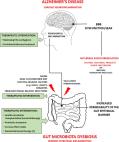
Alzheimer's disease (AD) is a neurodegenerative disease leading to dementia for which no effective medicine exists. Currently, the goal of therapy is only to slow down the inevitable progression of the disease and reduce some symptoms. AD causes the accumulation of proteins with the pathological structure of Aβ and tau and the induction of inflammation of nerves in the brain, which lead to the death of neurons. The activated microglial cells produce pro-inflammatory cytokines that induce a chronic inflammatory response and mediate synapse damage and the neuronal death. Neuroinflammation has been an often ignored aspect of ongoing AD research. There are more and more scientific papers taking into account the aspect of neuroinflammation in the pathogenesis of AD, although there are no unambiguous results regarding the impact of comorbidities or gender differences. This publication concerns a critical look at the role of inflammation in the progression of AD, based on the results of our own in vitro studies using model cell cultures and other researchers.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: found
The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—a Critical Review
- Record: found
- Abstract: found
- Article: found
Alzheimer’s disease prevention: from risk factors to early intervention

- Record: found
- Abstract: found
- Article: found